Protein Sciences Corp and UMN Pharma Inc are developing FluBlok an injectable trivalent influenza vaccine formulation composed of recombinant influenza hemagglutinin HA proteins that match the HA from the three influenza isolates that currently circulate in humans H1N1 H3N2 and B for the pote. Flublok Quadrivalent is available in packs containing I 5 or 10.
Flublok Quadrivalent also contains three times the active ingredients of other quadrivalent vaccines offer an advantage to people at high risk for flu complications such as seniors or those with lowered immunity.

What is flublok. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine FluBlok against culture-confirmed influenza in healthy adults. Flublok Quadrivalent is approved for use in persons 18 years of age and older see Clinical Studies 14. Find everything you need to know about Flublok Quadrivalent 2019-2020 including what it is used for warnings reviews side effects and interactions.
Flublok was a trivalent influenza vaccine first licensed by the FDA in 2013. For active immunization against disease caused by influenza A subtype viruses and influenza type B viruses contained in the vaccine. Vaccinations are used to prevent a number of infections conditions and diseases including chickenpox hepatitis A hepatitis B HPV meningitis pneumonia ear infections rabies typhoid rotavirus flu and shingles.
Formaldehyde a chemical typically present in the human body is a product of healthy digestive function. This method does not require an egg-grown vaccine virus and does not use chicken eggs in the production process. Flublok is part of the Vaccinations class and treats Flu Vaccination.
This vaccine is used to prevent infection by the influenza flu virus. Flublok has now been replaced by Flublok Quadrivalent which protects against four influenza strains instead of three. A single syringe contains 05 ml- of solution for injection.
Influenza can cause serious illness rarely death especially in people at high. August 19 2020 Influenza virus commonly known as the flu is a serious disease caused by a virus. FluBlok a recombinant trivalent hemagglutinin rHA vaccine produced in insect cell culture using the baculovirus expression system provides an attractive alternative to the current egg.
Flublok Quadrivalent is a clear and colourless solution. It is also called the seasonal flu shot. Flublok Quadrivalent Patient Group Direction PGD This PGD is for the administration of Flublok Quadrivalent recombinant quadrivalent influenza vaccine QIVr to individuals in accordance with the national influenza immunisation programme.
Flublok Quadrivalent is a vaccine indicated for active immunization against disease caused by influenza A subtype viruses and influenza type B viruses contained in the vaccine. In high doses formaldehyde is toxic and potentially lethal. For the 2020-2021 flu season there are two vaccines licensed for use that are manufactured without the use of eggs and are considered egg-free.
What Flublok Quadrivalent looks like and contents of the pack Flublok Quadrivalent is a solution for injection in a pre-filled syringe ready to use syringe. Flublok represents a new class of influenza vaccine and is the worlds first protein-based flu vaccine. FluBlok a next generation influenza vaccine manufactured in insect cells.
One recombinant influenza vaccine Flublok Quadrivalent four ingredient is available during the 20202021 influenza season. Flublok Quadrivalent 2020-2021 Intramuscular Last Updated. However the tiny amounts.
Flublok Quadrivalent licensed for use in adults 18 years and older Flucelvax Quadrivalent licensed for use in people 4 years and older. They work to build immunity and protection in the. A randomized placebo-controlled trial.
How is Flublok different than a traditional influenza vaccine. Flublok Quadrivalent was first licensed by the FDA in the United States for use in adults 18 years and older in 2017. It will be used as part of the UKs 202021 seasonal flu programme from.
Flublok is a vaccine indicated for immunization against influenza disease caused by influenza A and B strains contained in the vaccine. Flublok is a flu influenza vaccine for adults who are 18 years of age and older. Flublok Quadrivalent is a vaccine indicated for active immunization against disease caused by influenza A subtype viruses and type B viruses contained in the vaccine.
This PGD is for the administration of Flublok Quadrivalent vaccine by registered healthcare. Flublok Quadrivalent Dosage and Administration. An earlier trivalent version was licensed in 2013 but was later replaced by the quadrivalent version.
Influenza virus can spread from. FlublokFlublok Quadrivalent Recombinant influenza vaccines are produced using recombinant virus technology.
 Fda Approves Flublok Quadrivalent Flu Vaccine Pci
Fda Approves Flublok Quadrivalent Flu Vaccine Pci
 Vegan Flu Shots 2015 Update Ed V Food
Vegan Flu Shots 2015 Update Ed V Food
 Sanofi Ships First Flu Vaccines For 2018 2019 Season
Sanofi Ships First Flu Vaccines For 2018 2019 Season
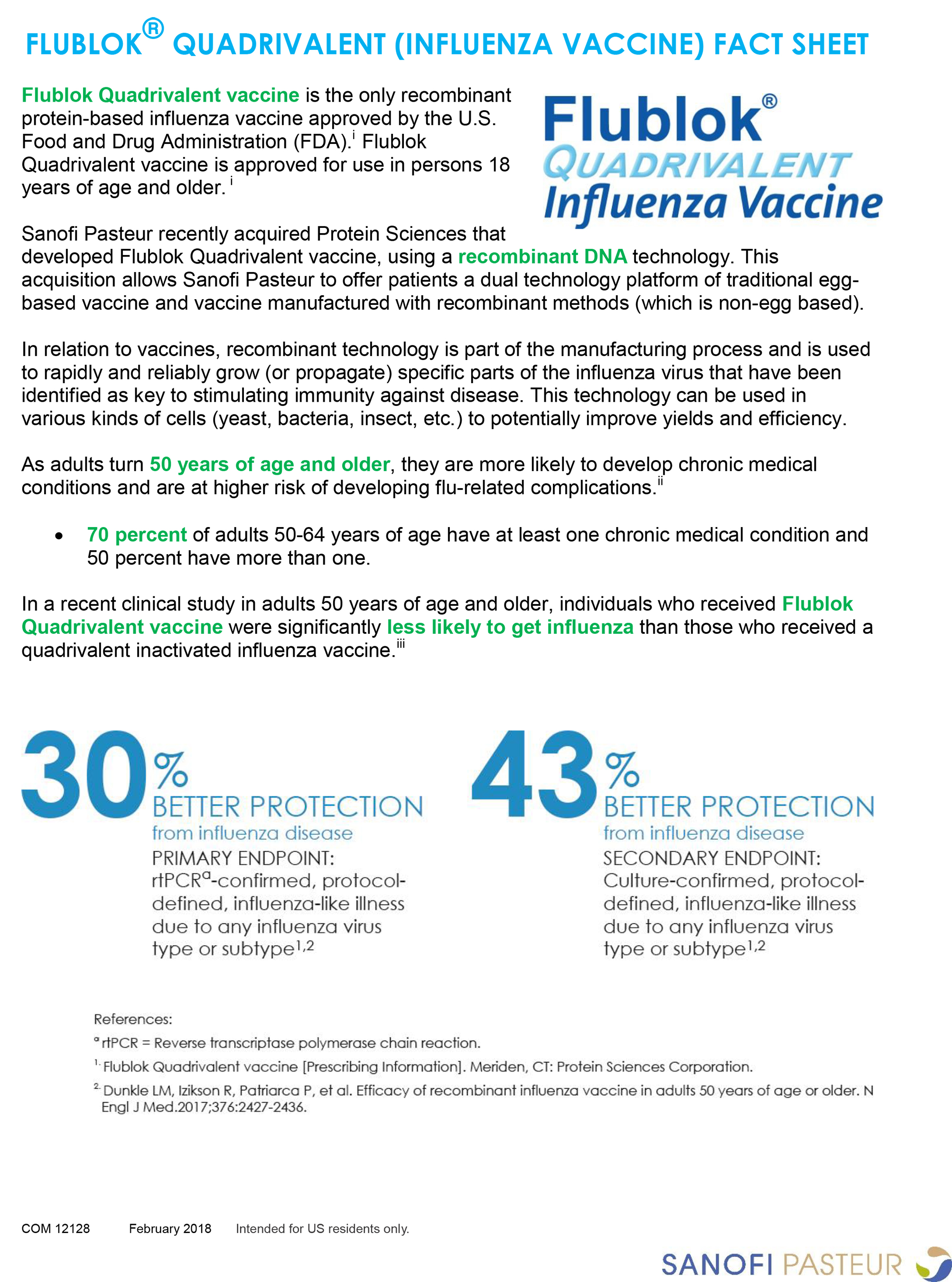 Sanofi Ships First Flu Vaccines For 2018 2019 Season Jul 30 2018
Sanofi Ships First Flu Vaccines For 2018 2019 Season Jul 30 2018
 Flublok Vaccine Guide National Vaccine Support Group
Flublok Vaccine Guide National Vaccine Support Group
 The Flublok Vaccine Available By Thanksgiving Valley Occupational Medical Center
The Flublok Vaccine Available By Thanksgiving Valley Occupational Medical Center
 Jacksonville Mayor Health Officials Kick Off Flu Shot Campaign
Jacksonville Mayor Health Officials Kick Off Flu Shot Campaign
Http Www Osc Ct Gov Empret Fluvacpgm 2016 Flublok 20info 20sheet Pdf
 Flublok Quadrivalent Influenza Vaccine Sanofi Flu
Flublok Quadrivalent Influenza Vaccine Sanofi Flu
 Flublok Vaccine Guide National Vaccine Support Group
Flublok Vaccine Guide National Vaccine Support Group
 Flu Vs Cold Fluzone High Dose Quadrivalent Influenza Vaccine And Flublok Quadrivalent Influenza Vaccine
Flu Vs Cold Fluzone High Dose Quadrivalent Influenza Vaccine And Flublok Quadrivalent Influenza Vaccine
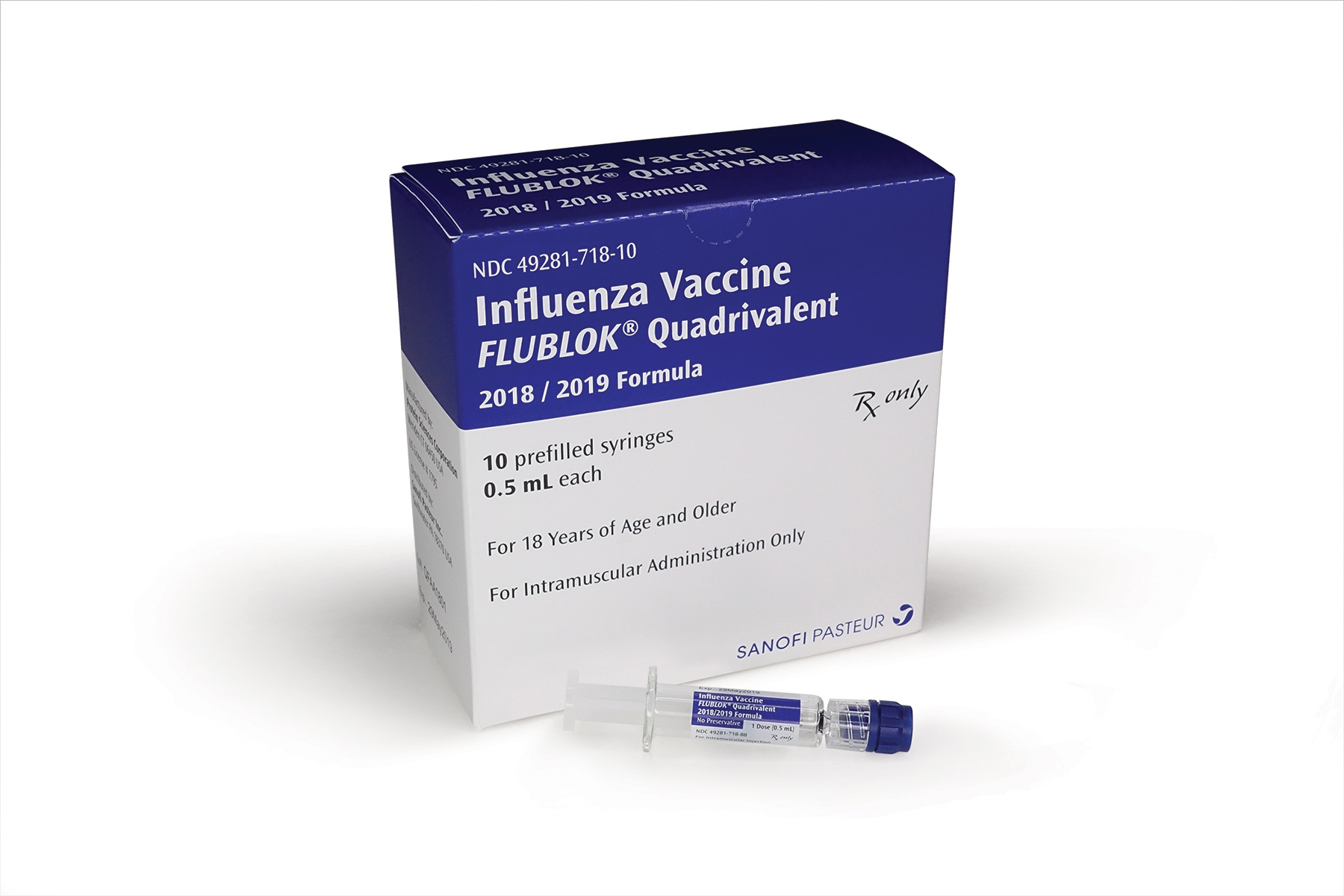 Sanofi Ships First Flu Vaccines For 2018 2019 Season
Sanofi Ships First Flu Vaccines For 2018 2019 Season
 Sanofi Pasteur 49281072010 Mckesson Medical Surgical
Sanofi Pasteur 49281072010 Mckesson Medical Surgical
 Flublok A New Type Of Flu Shot Vickery Vaccine Services
Flublok A New Type Of Flu Shot Vickery Vaccine Services


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.