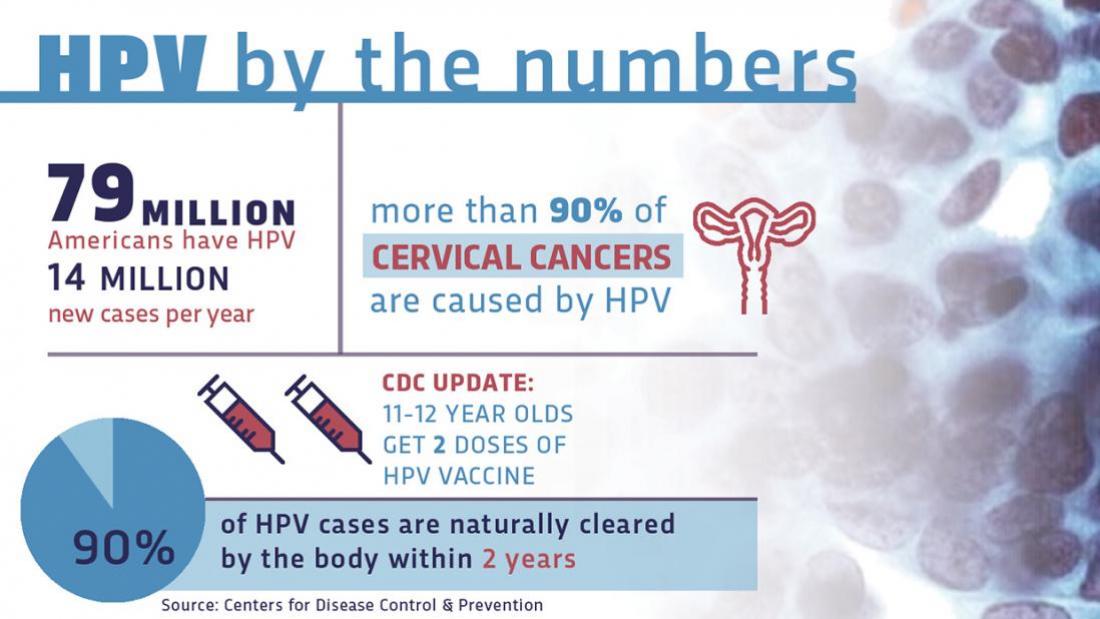
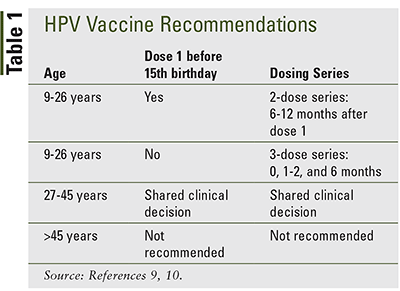
Started series at age 9 through 14 years except immunocompromised persons 2 0 6 to 12 months 5 months between doses Started series at age 15 through 26 years and immunocompromised persons any age 3 0 1 to 2 6 months 4 weeks between doses 1-2 12 weeks between doses 2-3 5 months. HPV vaccination is recommended for 11 and 12 year-old girls.
 Is The Hpv Vaccine Safe For My Child Siu School Of Medicine
Is The Hpv Vaccine Safe For My Child Siu School Of Medicine
The ACIP recommends that routine HPV vaccination be initiated for all children at age 11 or 12 years.
Hpv shot age. Evidence suggests that HPV vaccines are effective in preventing cervical cancer for women up to 45 years of age. The Advisory Committee on Immunization Practices ACIP recommends routine HPV vaccination at age 11 or 12 years. In June 2019 a key advisory committee for the US Centers for Disease Control and Prevention CDC recommended the vaccine for all men and women up to age 26.
If you miss either of your HPV vaccine doses speak to your school immunisation team or GP surgery and make an appointment to have the missed dose as soon as possible. Vaccination can be started as early as age 9 years. HPV vaccine can also be given to girls beginning at age 9 years.
The HPV Vaccine Schedule. Since the introduction of the Gardasil shot in 2006 it has inflamed fears about injury and promiscuity among young teens. Population of doses Routine schedule Minimum intervals.
Research has shown that receiving the vaccine at a young age isnt linked to an earlier start of sexual activity. In October 2018 the US Food and Drug Administration announced it had expanded the approved age for the HPV vaccine up to age 45 for women and men. It is also recommended for girls and women age 13 through 26 years of age who have not yet been vaccinated or completed the vaccine series.
The 1st dose of the HPV vaccine is routinely offered to girls and boys aged 12 and 13 in school Year 8. Only about 43 percent of teens ages 13. Human papillomavirus HPV is the most common sexually transmitted infection in the USA1 There are over 100 types of HPV approximately 15 of which are considered oncogenic high-risk types for the development of cervical cancer2 The US Food and Drug Administration has licensed three vaccines to prevent infection with HPV35 All licensed.
Its ideal for girls and boys to receive the vaccine before they have sexual contact and are exposed to HPV. Catch-up vaccination has been recommended through age 26 years. When should my child get the shot.
Health care experts say thats good news for women and men who did not receive the anti-cancer vaccine in childhood. Children who start the HPV vaccine series on or after their 15th birthday need three doses given over 6 months. Two doses of HPV vaccine are recommended for children at ages 1112.
The vaccine can be given starting at age 9 years. The Food and Drug Administration has raised the recommended age to receive the vaccine for human papillomavirus or HPV to 45. HPV vaccination is recommended at ages 11-12 to protect against cancers caused by HPV infection.
The Centers for Disease Control and Prevention CDC recommends HPV vaccine for all boys and girls ages 11 or 12 years old. It is an option for all men through age 21 but is recommended for men who have sex with men transgendered people or those who have a compromised immune system including HIV. Vaccination can be given starting at age 9 years.
The 2nd dose is offered 6 to 24 months after the 1st dose. If your teen hasnt gotten the vaccine yet talk to their doctor about getting it as soon as possible. The HPV vaccine is routinely recommended for girls and boys ages 11 or 12 although it can be given as early as age 9.
Vaccination is also recommended for all people age 13 through 26 years who have not been vaccinated previously or who have not completed the vaccination series. The vaccine is also recommended in teenage boys and girls who have not already received the vaccine or have not completed all booster shots. 11-12 Years 2 doses of the HPV shot are needed 6-12 months apart.
In 2018 the Food and Drug Administration released a summary basis for regulatory action and approval for expansion of usage and indication for the 9-valent HPV vaccine to include men and women 27 to 45 years of age. Guidelines recommend HPV vaccination for girls and boys at age 11 or 12 and catch-up vaccination for people through age 26 if they were not vaccinated when younger.


:max_bytes(150000):strip_icc()/how-long-are-rabies-shots-good-3385625__FINAL-5bedb5ba46e0fb00518ad5fe.png)
