Learn more about live attenuated vaccines. We describe the story of development licensing and implementation of live attenuated vaccines against varicella and zoster.
 Merck 00006496300 Mckesson Medical Surgical
Merck 00006496300 Mckesson Medical Surgical
A program of routine varicella vaccination of children 12-18 months of age begun in the United States in 1995 has been very successful in reducing the incidence of varicella.
Varicella zoster vaccine. Varicella is a highly contagious infection caused by varicella-zoster virus. Also a child between 12 months and 12 years of age might receive varicella vaccine together with MMR measles mumps and rubella vaccine in a single shot known as MMRV. Merck Co Inc Kenilworth NJ USA approved for the prevention of herpes zoster in healthy individuals is generally contraindicated in those who are immunocompromised.
The recombinant zoster vaccine RZV approved by the FDA in 2017 and Zoster Vaccine Live ZVL licensed in. One trial suggests 100 efficacy after 9 months and 98 after 2 years using a varicella vaccine with 17000 PFU at release. Vaccine is highly efficacious in preventing varicella disease in healthy children.
Another trial assessed vaccine efficacy to be 88 after an average of 29 months. Vaccine development was galvanised by the economic and societal burden of VZV including debilitating zoster complications that largely affect older individuals. As the vaccine is less stable than other live virus vaccines storage temperature requirements are critical to ensure optimum vaccine effectiveness.
Varicella also commonly referred to as chickenpox is an acute and highly contagious disease. The epidemiology of the disease differs. Can be given to children for their routine two doses of chickenpox vaccine at age 12 through 15 months and age 4 through 6 years.
Prevention of HZ through vaccination is a priority to avoid the significant burden of its incidence and complications. Kids who got chicken-pox vaccines are less likely to later get shingles. The preparation of master seed and stock seed lot are appropriately described in the dossier.
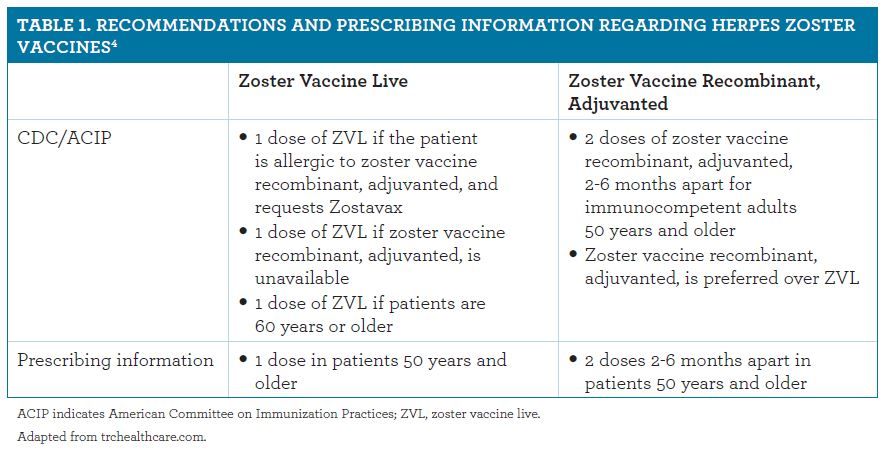
Conducted using VZV from the passage levels intended for commercial production varicella-zoster vaccine at the passage level for production was developed and evaluated in the setting of the applicants monovalent varicella vaccine VARIVAX. The Advisory Committee on Immunization Practices ACIP recommends a single dose of zoster vaccine for immunocompetent adults 60 years of age and older based on the. It causes a blister-like rash itching tiredness and fever.
Varicella vaccine Varivax 1400 pfu is the single-antigen varicella vaccine licensed in 1995 for use among healthy persons aged 12 months Proquad or MMRV 9800 pfu is a combination measles mumps rubella and varicella vaccine licensed in 2005 for use among healthy children aged 12 months-12 years CDC. Both vaccines contain live attenuated weakened varicella-zoster virus. Currently two HZ vaccines are available.
It is caused by primary infection with the varicella-zoster virus VZV. There are two chickenpox vaccines that are licensed in the United StatesVarivax and ProQuad. Chickenpox is a very contagious disease caused by the varicella-zoster virus VZV.
There are two chickenpox vaccines approved for use in the United States. Older children adolescents and adults also need 2 doses of varicella vaccine if they are not already immune to chickenpox. Is licensed for use in children age 12 months and older adolescents and adults.
The weakened varicella zoster virus strain in vaccines also lurks dormant in neurons but it does not reawaken so easily. Who Should Get Chickenpox Vaccine. Varicella occurs worldwide and in the absence of a vaccination programme affects nearly every person by mid-adulthood.
One single antigen vaccine and one combination vaccine. Varicella- Zoster arch 2020 varicella vaccine is administered vaccine. A live attenuated varicella zoster vaccine Zostavax zoster vaccine live.
Varicella-containing vaccine is recommended for children at 18 months of age as MMRV measles-mumps-rubella-varicella vaccine. Primary infection with varicella-zoster virus causes varicella chickenpox. Varicella vaccine may be given at the same time as other vaccines.
Varivax Contains only chickenpox vaccine. CDC recommends two doses of chickenpox vaccine for children adolescents and adults. There is no increased risk if varicella vaccine is given 4 weeks after MMR vaccine.
