Isopropyl alcohol CH3CHOHCH3 or CH32CHOH or C3H8O CID 3776 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. The IUPAC functional class name is isopropyl alcohol and the substitutive name is isopropanol.
 Toxic Alcohols 101 Ethanol Methanol Isopropanol Berkshire Corporation
Toxic Alcohols 101 Ethanol Methanol Isopropanol Berkshire Corporation
Isopropyl alcohol was a compromise.

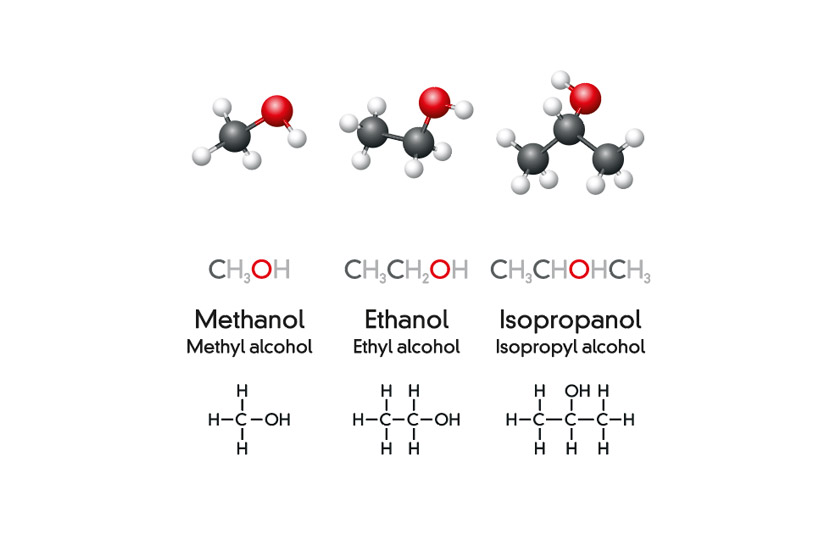
Isopropyl vs isopropanol. The distinction between ethanol and isopropyl alcohol is pretty simple. Hence the key difference between 2 propanol and isopropanol is that the 2 propanol is the IUPAC name for the compound having the chemical formula C 3 H 8 O whereas the isopropanol is the common name for the same compound. They both play an essential role in killing bacteria and are at a concentration of 70 water.
As you can see both types of alcohol are effective at killing various species of bacteria. Isopropyl alcohol is known as isopropanol or rubbing alcohol. However these are both general names since the iso prefix is from the common naming system.
Yes they are the same and a chemist would not be confused by either name. Ethanol works by dissolving lipids and denaturing proteins. In other cases isopropyl alcohol is more effective than ethanol at lower percentage concentrations.
Isopropyl alcohol 99 percent is pure isopropanol and isopropyl alcohol 70 percent is pure isopropanol diluted with 30 percent purified water by volume explains Dr. Most know isopropyl alcohol as the household rubbing alcohol. Most know isopropyl alcohol as the household rubbing alcohol.
Ethanol is stable and isopropyl is not stable. Under the IUPAC system the correct name is propan-2-ol although it is not as commonly used as either isopropyl alcohol or isopropanol. N-propanol will not work as well for a few reasons.
DNA is less soluble in isopropanol so it precipitates faster even at low concentrations. A familiar component in the cleanroom IPA is the abbreviation of isopropyl alcohol aka isopropanol 2-propanol or rubbing alcohol Its chief claim to fame especially now in the time of the pandemic is as the active ingredient in hand sanitizer. Thusonly the terminology is different.
The downside however is that salt will also precipitate in isopropanol. Ethanol however is more effective at 70 90 concentrations against FCV. Ethanol which is also known as ethyl alcohol is present in alcoholic beverages.
Isopropyl alcohol is fully trivial. However ethanol is usually a little more drying to the skin so it may be more uncomfortable to use a hand sanitizer made with ethyl alcohol instead of isopropyl alcohol. Isopropyl Alcohol also known as IPA or Isopropanol is a synthetic alcohol made from propene which is derived from crude oil.
Isopropyl vs Ethanol Ethanol is an organic molecule and isopropyl is only a part of organic molecule. Its a mash-up of IUPAC the -ol suffix and trivial the iso- prefix. With ethanol the DNA needs to be at a higher concentration to flocculate but the salt tends to stay soluble even at colder temperatures.
Lucky Sekhon fertility specialist and board certified OBGYN in New York City. Isopropyl Alcohol Wikipedia Wikimedia Foundation 13 Oct. But isopropanol is strictly speaking incorrect nomenclature.
Isopropyl alcohol is an antiseptic that contains denaturants that make it dangerous for human consumption whereas ethanol is the only type of alcohol safe for human consumption and contains no toxic impurities. In contrast isopropyl alcohol evaporates much. The deaths associated isopropanol intoxication are mainly due to the peoples lack of knowledge how intoxicating the substance is compared to ethanol.
Isopropyl Alcohol or Isopropanol is more potent than Ethanol the alcohol found in alcoholic beverages. It is slower to evaporate and will act like the butyl cellosolves not that slow of the current alcohol substitutes. Isopropyl alcohol is effective against viruses such as FCV at 40 60 concentrations.
Isopropyl has three carbon atoms and ethanol has only two carbon atoms. Also I think it will be a better solvent for the ink vehicles and thus tend to.
