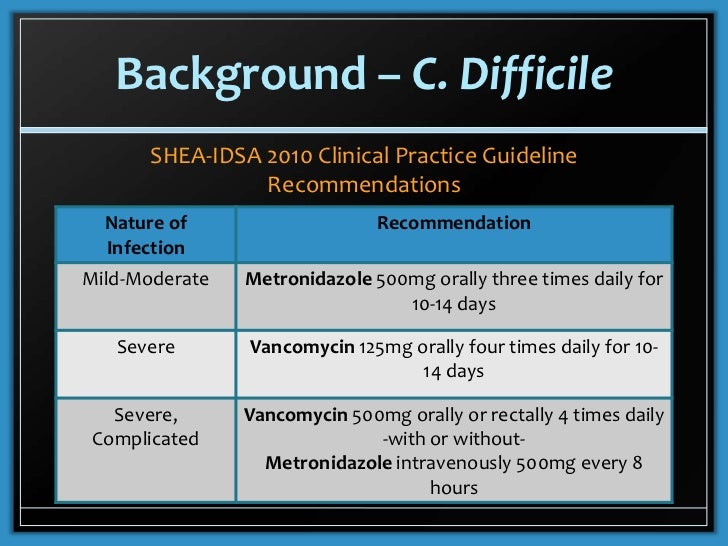
For the treatment of Clostridioides difficile-associated diarrhea CDAD Usual Pediatric Dose for Clostridial Infection. However pediatric data are limited.
 Vancomycin Versus Fidaxomicin Effects On Time To Recurrence Of Download Table
Vancomycin Versus Fidaxomicin Effects On Time To Recurrence Of Download Table
120 mg orally twice a day-Weight 9 to less than 125 kg.
Fidaxomicin c diff. What do I need to tell my doctor BEFORE I take Fidaxomicin. Fidaxomicin a narrow-spectrum antibiotic approved for Clostridioides Clostridium difficile infection CDI in adults is associated with lower rates of recurrence than vancomycin. This multicenter investigator-blind phase 3 parallel-group trial assessed the safety and efficacy of fidaxomicin in children.
Fidaxomicin trade name Dificid is a new type of antibiotic. Fidaxomicin is an antibiotic specifically used for the treatment of Clostridium difficile. Developmental and health benefits of.
Diff bacteria can be found on furniture bathroom floors telephones fingernails jewelry toilet seats and other places. This in turn lessens the chance of further C Diff attacks. However not all people infected with C.
At first the use of fidaxomicin for the treatment of Clostridioides difficile C diff remained low but after a release of guidelines and relaxing of system restrictions the use of fidaxomicin increased according to a poster presented during IDWeek 2019. 160 mg orally twice a day. The other from 163 to 31remarkable decreases.
There is no information on presence of fidaxomicin or its main metabolite OP-1118 in human milk the effects on breastfed infant or on milk production. 80 mg orally twice a day-Weight 7 to less than 9 kg. It kills the bacteria causing the infection and this helps to reduce the diarrhoea associated with the infection.
It kills the target bacteria and has been shown to kill Clostridium difficile without attacking the many healthy bacteria found in the normal healthy intestine. It has no activity against gram-negative. Oral suspension-Weight 4 to less than 7 kg.
Diff infection are fever abdominal pain and cramps. Study findings are consistent with current treatment guideline recommendations for the use of either agent in the management of severe CDI. Summary Fidaxomicin a macrocyclic antibiotic has a narrow spectrum of activity against gram-positive anaerobes and is bactericidal against C.
Cdifficile colitis recurrence is defined as recurrent symptoms and positive testing after initial resolution 8 weeks from the start of the original episode 4. Courses of fidaxomicin or oral vancomycin for severe CDI resulted in similar treatment outcomes. Diff are antibiotics and surgery in some cases.
Fidaxomicin Dificlir is the first in a new class of macrocylic antibiotics and has recently been licensed by the European Medicines Agency EMA for the treatment of Clostridium difficile infection CDI. In 2 health care systems that decided to use fidaxomicin broadly meaning every patient that had C difficile 1 health care systems recurrence rate went from 106 to 31. Compared with vancomycin fidaxomicin use was associated with a significantly lower recurrence of CDI with a pooled OR of 047 95 confidence interval CI 037 - 060 I2 0.
Parenteral administration of metronidazole has poor intraluminal penetration and should not be used alone for treatment. Fidaxomicin sold under the brand name Dificid among others is the first member of a class of narrow spectrum macrocyclic antibiotic drugs called tiacumicins. Fidaxomicin versus oral vancomycin for severe Clostridium difficile infection.
Current Health Protection Agency HPA guidance recommends metronidazole as first-line therapy in mild to moderate CDI but vancomycin in severe cases. It is a fermentation product obtained from the actinomycete Dactylosporangium aurantiacum subspecies hamdenesis. Fidaxomicin fye DAX oh mye sin Brand Name.
A retrospective cohort study. It is used to treat diarrhea caused by a bacterial infection called C diff. 6 Months to Less Than 18 Years.
Extended-pulsed fidaxomicin was superior to standard-dose vancomycin for sustained cure of C difficile infection and to our knowledge extended-pulsed fidaxomicin recurrence rates in this study are the lowest observed in a randomised clinical trial of antibiotic treatment for C difficile infection. It is taken orally and has a low impact on the bloodstream.
