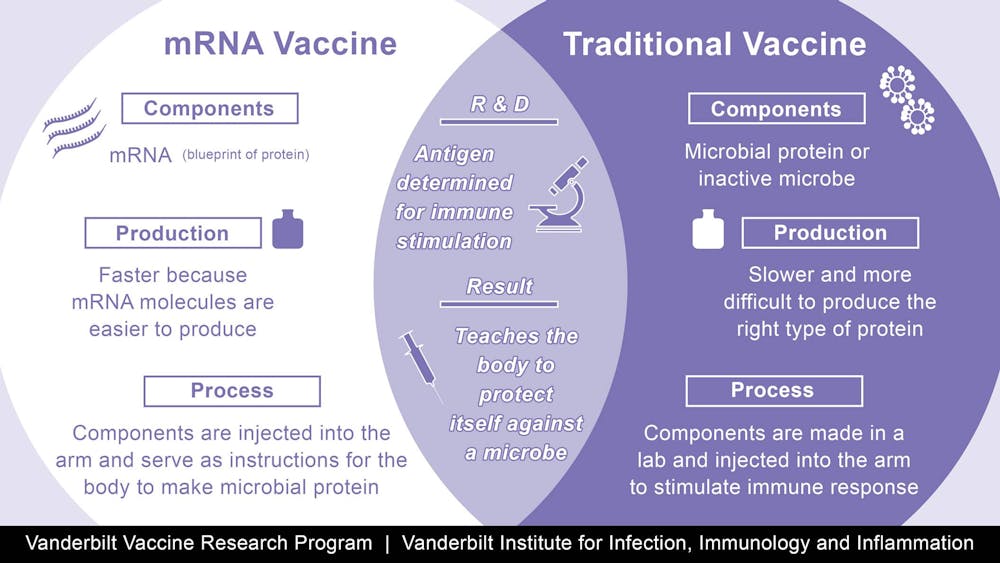
There are currently two mRNA vaccines. These vaccines work by.
 A Thermostable Mrna Vaccine Against Covid 19 Sciencedirect
A Thermostable Mrna Vaccine Against Covid 19 Sciencedirect
Novavax still in clinical trials How it works.

What vaccines use mrna. This is whats currently used in the Hepatitis V and one of the shingles vaccines Shingrix. MRNA vaccines are a new type of vaccine to protect against infectious diseases. It is enclosed in nanoparticle lipid calledMatrix-M.
Messenger RNA vaccinesalso called mRNA vaccinesare some of the first COVID-19 vaccines authorized for use in the United States. Many types of vaccines use a weakened or inactivated virus or part of a virus to trigger an immune response inside our body. CUREVACGLAXOSMITHKLINE Germany-based CureVac NV is working on mRNA-based vaccines.
The new mRNA vaccine was developed in haste and had never been used on a large scale for the prevention of infectious disease and its safety had not been confirmed for large-scale use. Brosh compared the mRNA vaccine to traditional vaccines such as those for influenza which use an inactivated virus that was destroyed by heat or. The Pfizer-BioNTech vaccine was approved earlier this month for emergency use in the US UK and Canada.
Viral Vector COVID-19 Vaccines Information about viral vector vaccines generally and COVID-19 vaccines that use this new technology specifically. Many of the vaccines developed to protect against COVID-19 are forms of messenger RNA mRNA vaccines. Both of these vaccines require 2 doses to be effective.
On the flip side the Moderna and PfizerBioNTech vaccines which send mRNA into muscle cells to tell the body to produce an immune reaction to the coronavirus spike protein do seem to have higher. It has signed a collaboration agreement with GSK to. Not well understood 12 life in this setting.
An mRNA vaccine encodes proteins of a virus which is inserted into a cell to trigger an immune response and create antibodies There has never. Vaccines that use this. Sahin and colleagues have pioneered the use of individualized neoepitope mRNA cancer vaccines 121.
Most vaccines for SARS-CoV-2 provoke an immune response that targets the coronavirus spike protein which is found on the surface of the virus. Never before used in humans. Vaccines in Phase 3 Clinical Trials.
However instead of using the live virus that causes COVID-19 mRNA vaccines teach our cells how to make a. Some of the vaccine candidates that are most advanced in development are messenger RNA vaccines called mRNA vaccines. The vaccine mRNA doesnt intercalate into your DNA but mRNA is an epigenetic controller of DNA.
Information about mRNA vaccines generally and COVID-19 vaccines that use this new technology specifically. Messenger RNA vaccines encode segments of the spike protein and those mRNA sequences are much easier to. Not to mention saponins that are part of Matrix M.
New Approach to Vaccines. The vaccines essentially work by. They use high-throughput sequencing to identify every unique somatic mutation of.
The Moderna and PfizerBioNTech vaccines are forms of mRNA vaccine. The Pfizer vaccine and the Moderna vaccine use synthetic mRNA that contains information about the coronaviruss signature spike protein. To trigger an immune response many vaccines put a weakened or inactivated germ into our bodies.
And how fo you know how long the nRNA lasts. MRNA vaccines are a new type of vaccine.