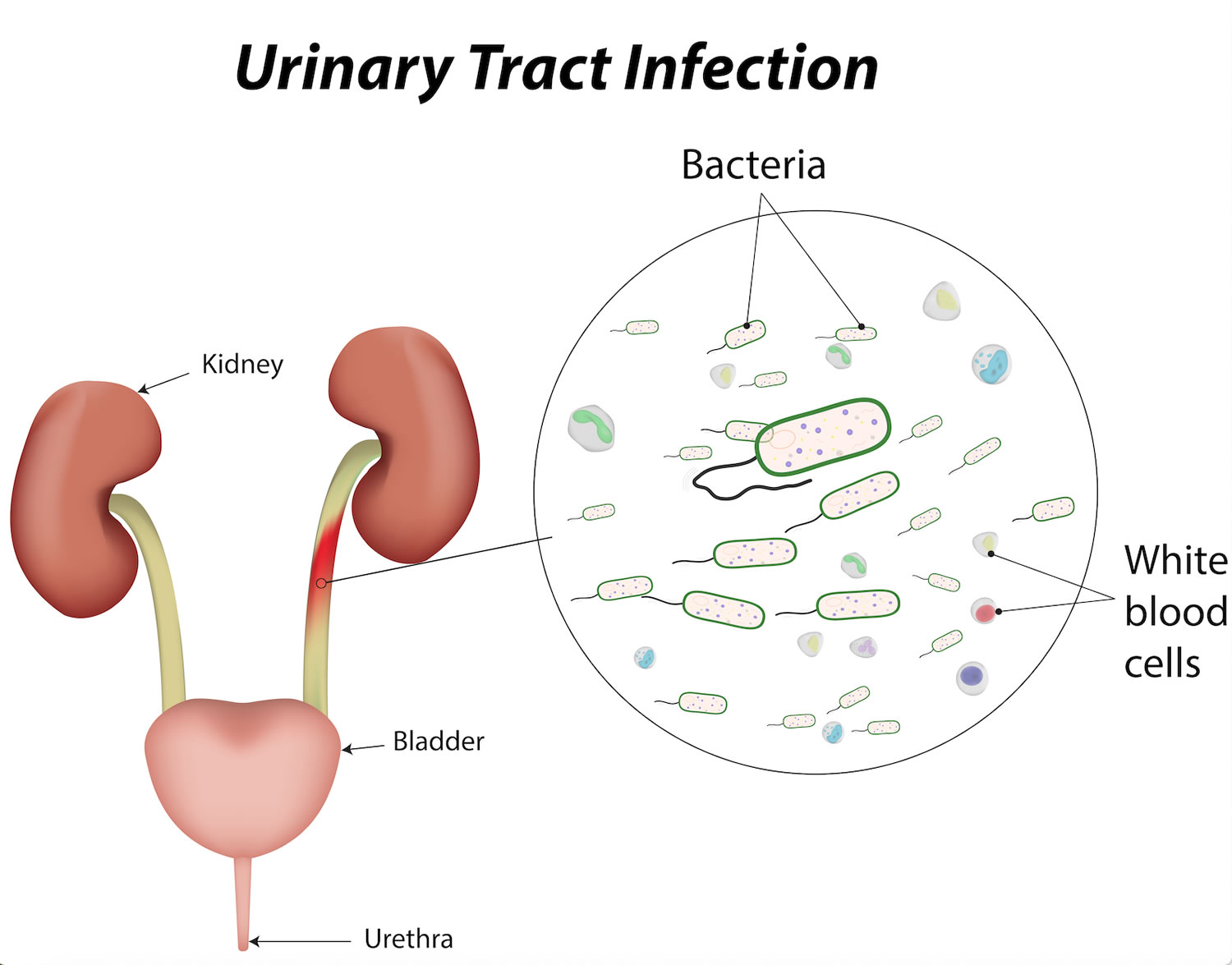
See 21 CFR part 207 The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib Xeljanz Xeljanz XR 02042021.
 List Of Fda Approved Drugs That Form Covalent Interactions With Targets Download Table
List Of Fda Approved Drugs That Form Covalent Interactions With Targets Download Table
Over the past several weeks the FDA has worked in consultation with other federal partners to develop a list of 227 drug and biological product essential medicines and medical countermeasures.

Fda drug list. EmtricitabineTenofovir Disoproxil Fumarate. It includes detailed notes on the clinical pharmacology of a wide variety of drugs. Search list of.
FDA provides a searchable list of recalled products. Purple Book database of FDA-licensed approved biological products including biosimilar and interchangeable. Product-Specific Guidances for Generic Drug Development Database.
158 rows Drug Name Submission Active Ingredients Company Submission Classification. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring MD 20993 1-888-INFO-FDA 1-888-463-6332 Contact FDA. The FDA approved one drug and many more are being tested in clinical trials Recent News The FDA is committed to bringing new safe and effective treatment options like Opdivo to patients with.
Drug recalls are actions taken by a firm to remove a product from the market and may be conducted on a firms own initiative by FDA request. The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration FDA. Click for detailed instructions.
This list is not limited to drugs that were ever approved by the FDA. You need to enable JavaScript to run this app. FDA Professional Drug Information The Professional Drug Information database is a repository of drug information sourced directly from the FDA.
53 rows Here is a list of some significant drug withdrawals. Some of them Lumiracoxib Rimonabant Tolrestat Ximelagatran and Zimelidine for example were. Tenofovir Disoproxil Fumarate ANDA 208740.
Qelbree FDA Approval History. Qelbree viloxazine hydrochloride is a serotonin norepinephrine modulating agent SNMA for the treatment of attention deficit hyperactivity disorder ADHD in pediatric patients 6 to 17 years of age. The list of DMFs which is updated quarterly contains DMFs RECEIVED by December 31 2020 for which acknowledgment letters were sent before January 11 2020.
This page searches the Orphan Drug Product designation database. You need to enable JavaScript to run this app. Results can be displayed as a condensed list detailed list or an Excel spreadsheet.
FDA Approves Qelbree viloxazine for the Treatment of ADHD - April 2 2021. Searches may be run by entering the product name orphan designation and dates. Drug Name and Application Number Active Ingredient Dosage Form Route Submission Company Submission Classification Submission Status.
DrugsFDA includes information about drugs including biological products approved for human use in the United States see FAQ but does not include information about FDA-approved products regulated by the Center for Biologics Evaluation and Research for example vaccines allergenic products blood and blood products plasma derivatives cellular and gene therapy products.